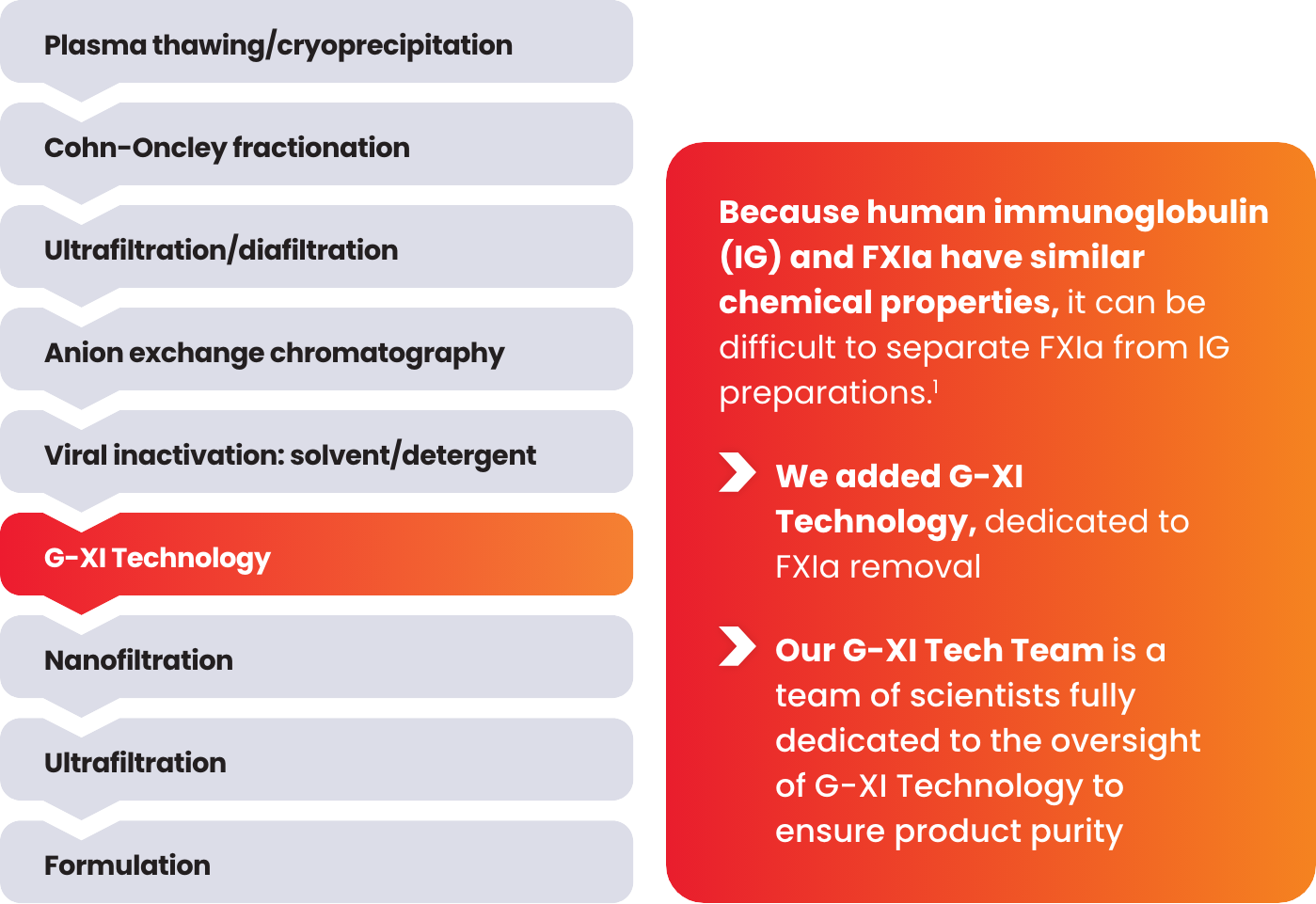
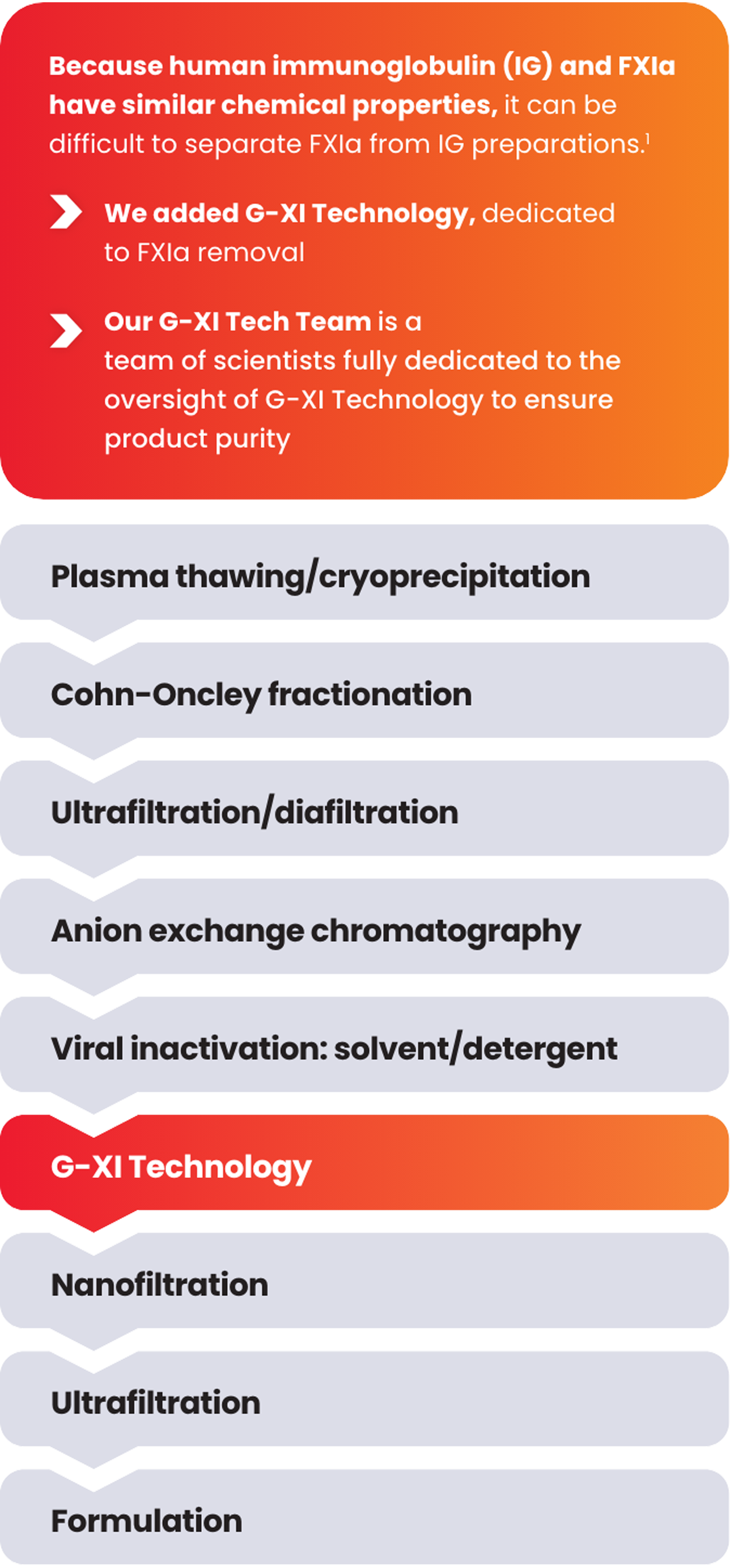
Product end-testing is conducted to help ensure undetectable levels of FXIa in every lot.
During this step, cation exchange (CEX) chromatography is implemented using a unique ceramic resin under specific conditions that aid in the removal of FXIa.1

FXla remains
Residual activated coagulation factor XI remained in the solution.1
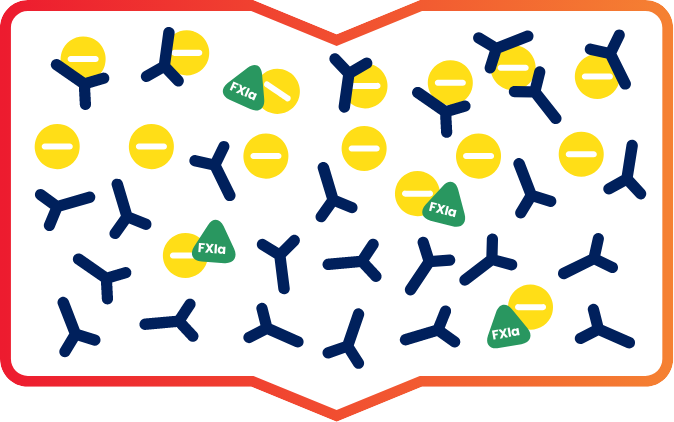
Positively charged IG and FXla initially bind to the negatively charged ceramic resin.1
Then, under specific conditions, IG flows through while the FXIa remains bound to the resin, enabling IG to effectively separate from FXIa.1
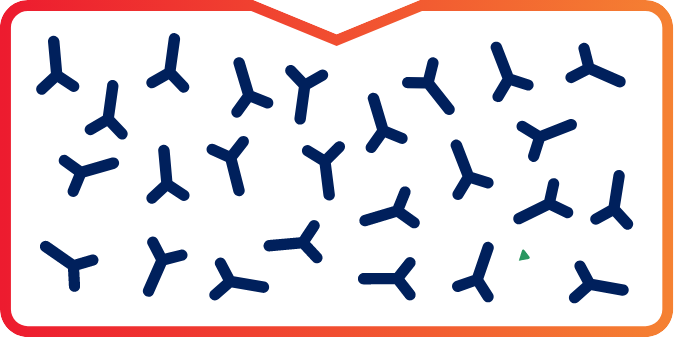
FXla FREE
Once IG is collected, FXIa levels are undetectable in the final intravenous immunoglobulin (IVIG) preparations.1
Representation of undetectable limits.

IgG, immunoglobulin G.
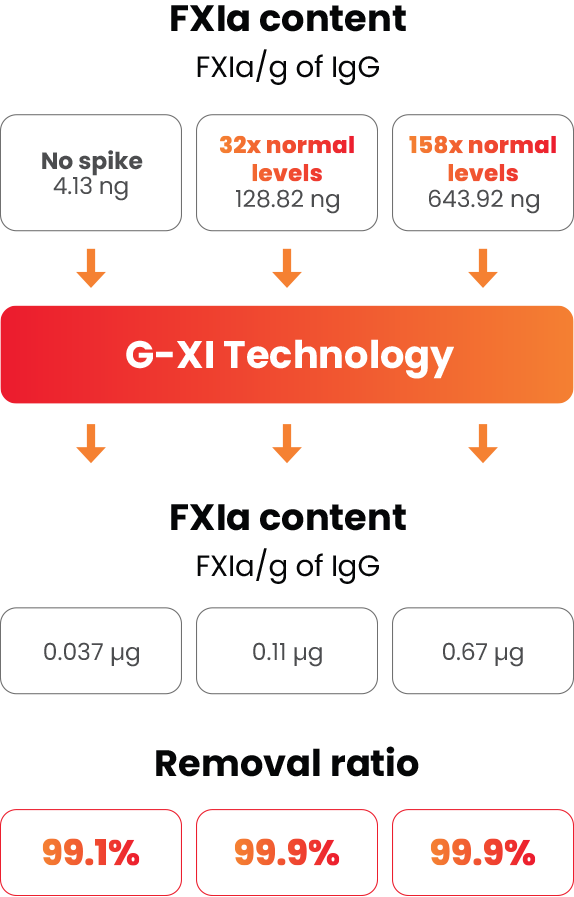
G-XI Technology was proven to remove >99% of FXIa in a published, multi-step study1:
Measured coagulation factor levels at each phase of the manufacturing process
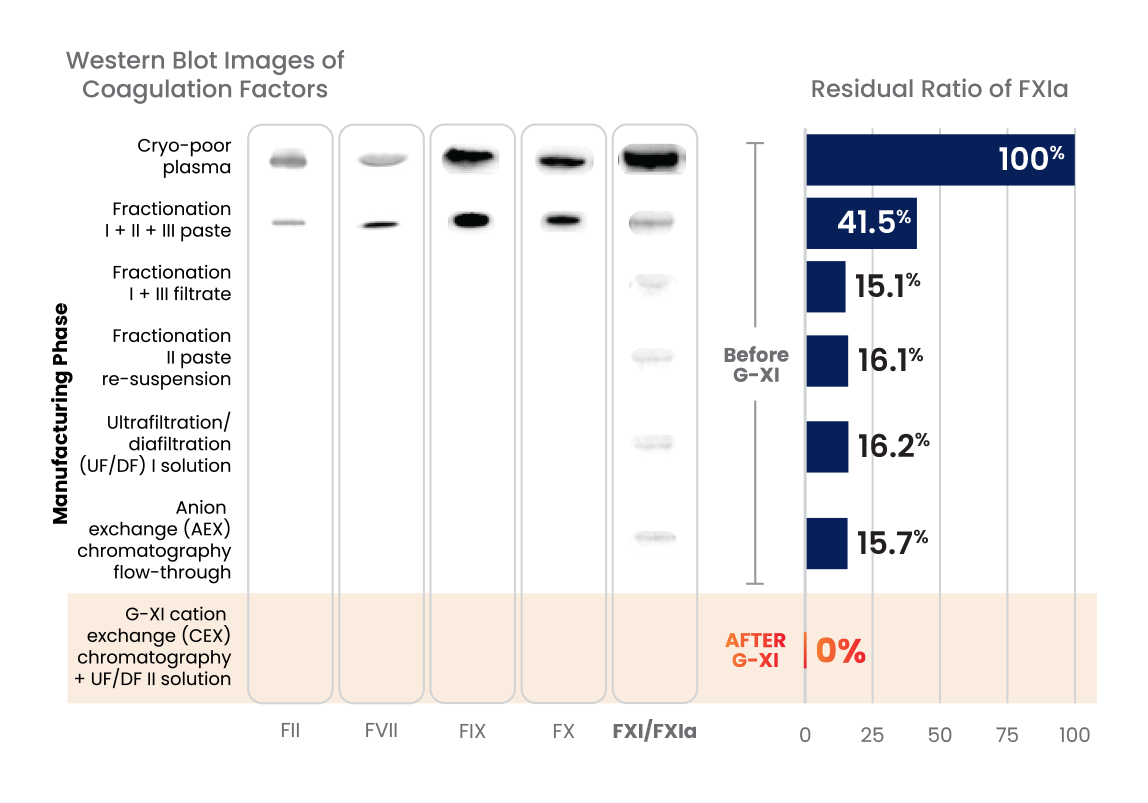
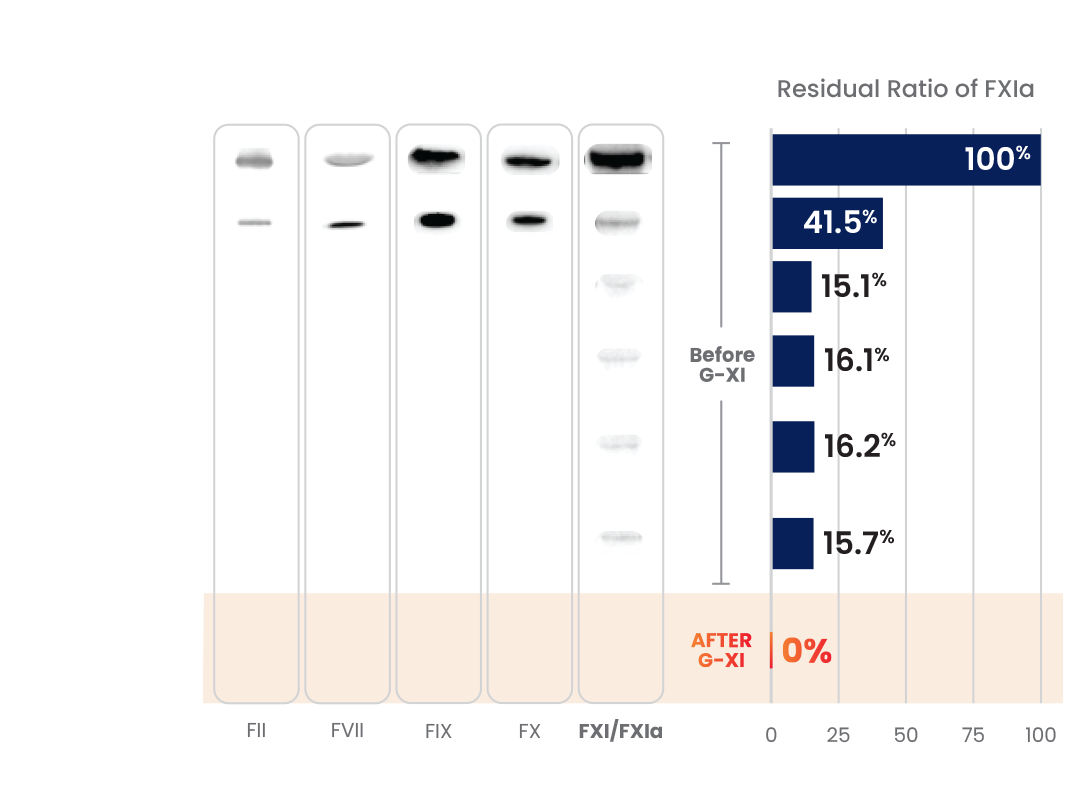

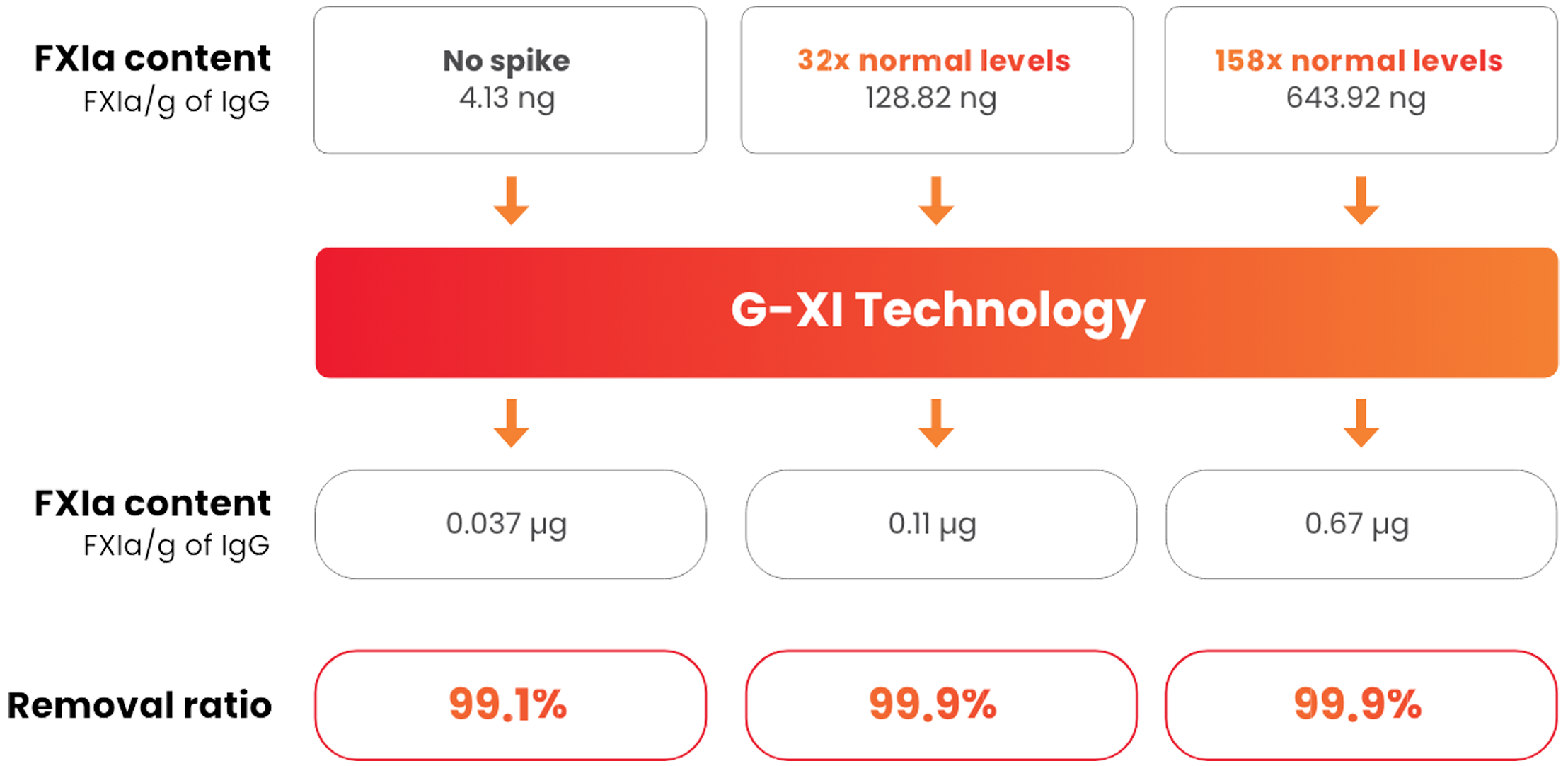
Samples were spiked with large quantities of FXIa (32-fold and 158-fold) prior to the step of G-XI Technology, and assays were used to confirm FXIa levels.1

Reference:
ALYGLO® is indicated for the treatment of primary humoral immunodeficiency (PI) in adults aged 17 years and older. This includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency (CVID), Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
Thrombosis may occur with immune globulin intravenous (IGIV) products, including ALYGLO. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
Renal dysfunction, acute renal failure, osmotic nephropathy, and death may occur with the administration of IGIV products in predisposed patients.
Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. ALYGLO does not contain sucrose.
For patients at risk of thrombosis, renal dysfunction or renal failure, administer ALYGLO at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
For more information about ALYGLO, please see full Prescribing Information.