Demand for IG replacement is outpacing supply.1
IVIG, intravenous immunoglobulin;
PI, primary immunodeficiency.
Global demand for IG treatment is increasing 6-8% per year,1 driven by:
Increasing recognition
and diagnosis of PI1
The chronic
nature of PI1
Use of IVIG for other
disease states1
At times, supply shortages have resulted in changes to patient prioritization and infusion schedules.2 That’s why more IVIG products are critical to ensuring access to treatment for patients with PI.2
While IG treatment is the gold standard of care for patients with PI and several other diseases,2 thromboembolic events (TEEs) are a rare but serious potential adverse event.3
FXIa, activated coagulation factor XI.
FXIa has been identified as one of the root causes of IVIG-related TEEs.8
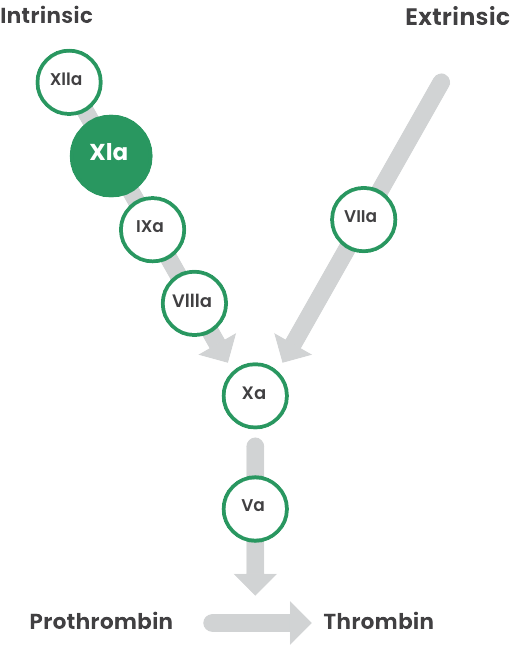
In 2010, the U.S. Food and Drug Administration (FDA) became aware of a cluster of TEEs associated with FXIa in IVIG8
In 2013, this led the FDA to place a mandatory boxed warning for risk of TEEs in all IG products10
Regulatory bodies across the world have implemented corrective actions, encouraging manufacturers to test products at lot release and to consider dedicated steps for removing FXIa8
At GC Biopharma, we have implemented the extra step of G-XI™ Technology, which utilizes cation exchange (CEX) chromatography, to help reduce FXIa levels below detectable limits3
A longstanding goal within the IG industry has been to remove FXIa, a plasma-based protein linked to IVIG-related thromboembolic events.3 But separating it in manufacturing can be a challenge.
The manufacturing process for ALYGLO includes multiple steps, such as Cohn-Oncley cold ethanol fractionation, viral inactivation, and nanofiltration.3
A study demonstrated that coagulation factors II, VII, IX, and X are effectively removed during these steps, but residual FXI/FXIa remains in the intermediate solution.3
Since human IG and FXIa share a similar isoelectric point (pI), it can be difficult to separate FXIa from IG preparations using ethanol precipitation alone.3
Human IG: 6.4-9 pI
FXIa: 8.9-9.1 pI
References:
ALYGLO® is indicated for the treatment of primary humoral immunodeficiency (PI) in adults aged 17 years and older. This includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency (CVID), Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
Thrombosis may occur with immune globulin intravenous (IGIV) products, including ALYGLO. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
Renal dysfunction, acute renal failure, osmotic nephropathy, and death may occur with the administration of IGIV products in predisposed patients.
Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. ALYGLO does not contain sucrose.
For patients at risk of thrombosis, renal dysfunction or renal failure, administer ALYGLO at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
For more information about ALYGLO, please see full Prescribing Information.