
Consider ALYGLO for adult patients aged 17 and older.
PI, primary immunodeficiency.

Consider ALYGLO for patients who are seeking intravenous immunoglobulin (IVIG) with undetectable levels of coagulation factor XI (FXIa).

Consider ALYGLO for patients who are looking for IVIG treatment administered by a nurse at home or in an infusion clinic.
ALYGLO is infused every 3-4 weeks administered by a nurse in the patient’s home or at an infusion clinic.
Allow ALYGLO to reach room temperature prior to administration.
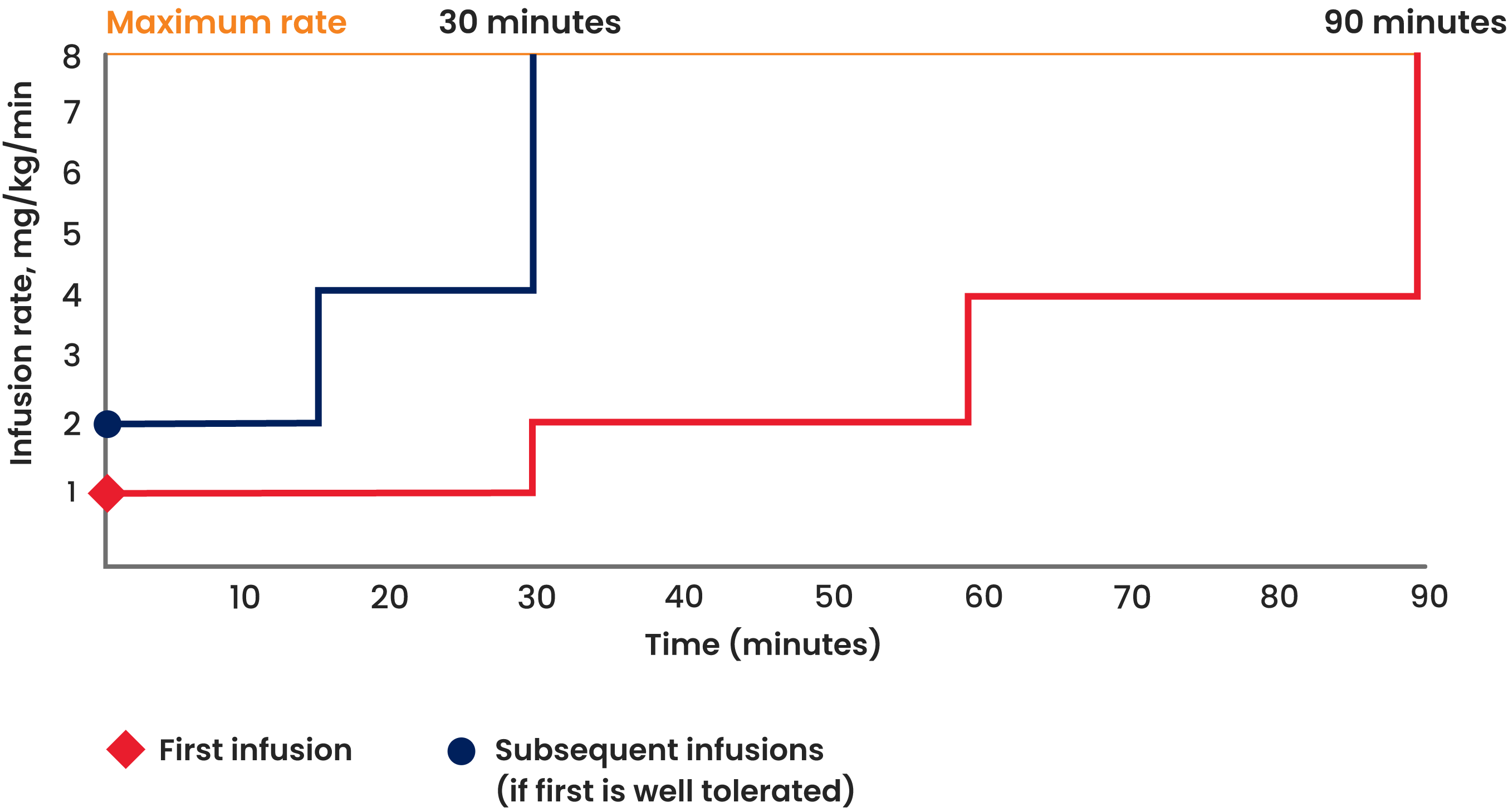
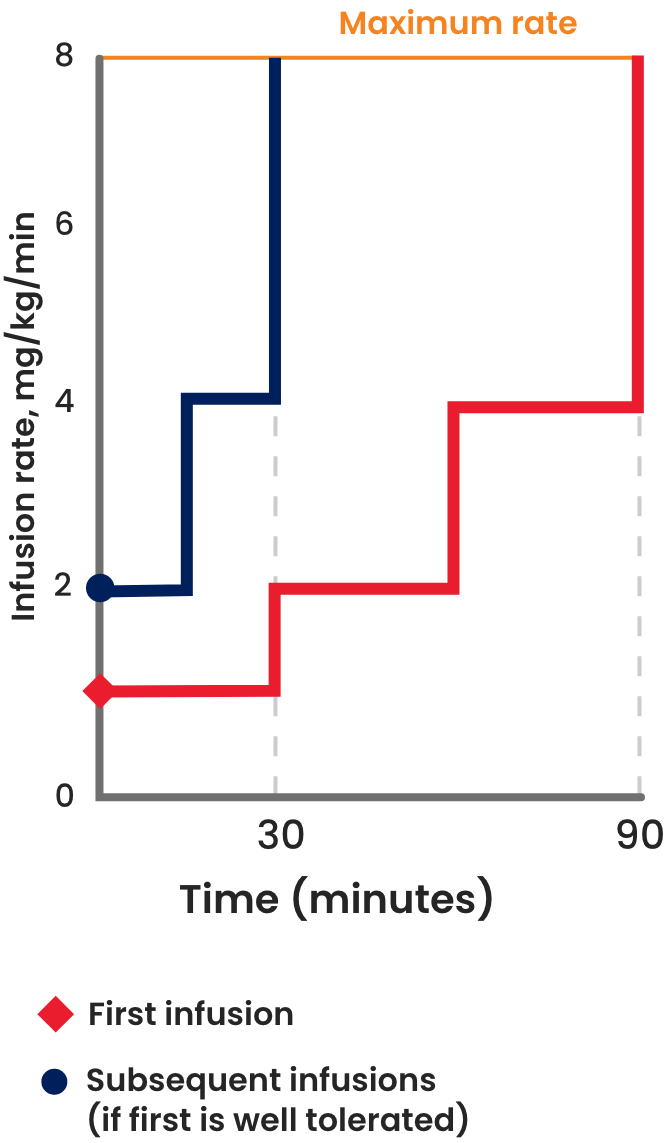
After the initial infusion, the maintenance infusion rate of ALYGLO can be doubled every 15 minutes to reach the maximum rate if well tolerated. This can help shorten the overall infusion time for your patients.
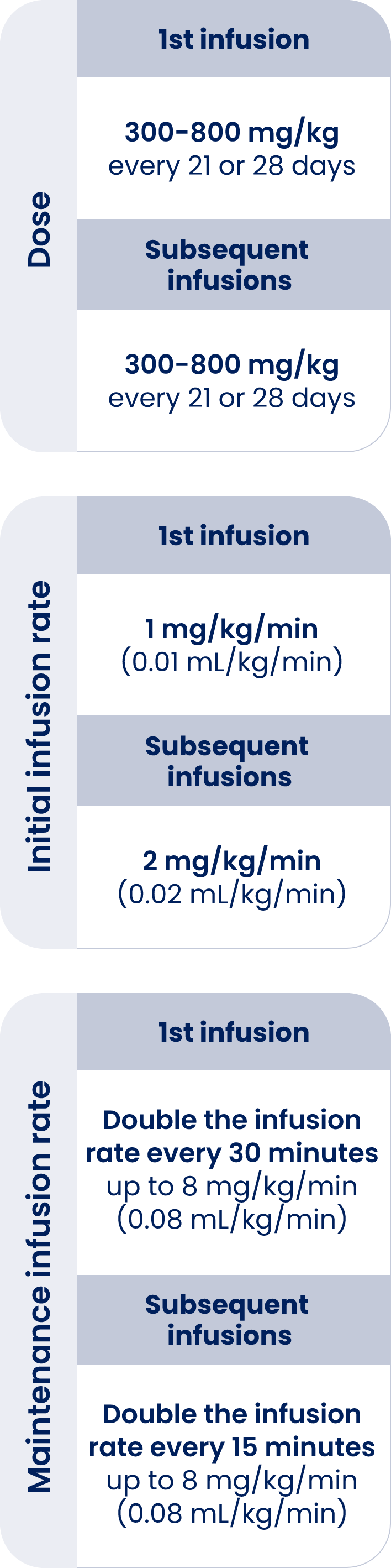
| 1st infusion | Subsequent infusions | |
|---|---|---|
| Dose | 300-800 mg/kg
every 21 or 28 days |
300-800 mg/kg
every 21 or 28 days |
| Initial
infusion rate |
1 mg/kg/min
(0.01 mL/kg/min) |
2 mg/kg/min
(0.02 mL/kg/min) |
| Maintenance
infusion rate |
Double the infusion rate every 30 minutes up to 8 mg/kg/min (0.08 mL/kg/min) |
Double the infusion rate every 15 minutes up to 8 mg/kg/min (0.08 mL/kg/min) |
For more detailed clinical information on product administration, please contact 1-833-426-6426 or email medicalinfo@gcbiopharmausa.com
5, 10, and 20-gram recyclable vials
References:
ALYGLO® is indicated for the treatment of primary humoral immunodeficiency (PI) in adults aged 17 years and older. This includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency (CVID), Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
Thrombosis may occur with immune globulin intravenous (IGIV) products, including ALYGLO. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
Renal dysfunction, acute renal failure, osmotic nephropathy, and death may occur with the administration of IGIV products in predisposed patients.
Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. ALYGLO does not contain sucrose.
For patients at risk of thrombosis, renal dysfunction or renal failure, administer ALYGLO at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
For more information about ALYGLO, please see full Prescribing Information.